The Role of Genetics in Pain Sensitivity and Management
Pain, a universal human experience, varies greatly among individuals. While some may shrug off discomfort with ease, others find themselves grappling with debilitating agony even in response to seemingly minor stimuli. This diversity in pain sensitivity has long intrigued researchers, prompting investigations into the underlying factors that shape our perception of pain.
Among these factors, genetics emerges as a significant player, influencing not only how keenly we feel pain but also how we respond to various forms of treatment. Every individual possesses a unique genetic makeup, and subtle variations within our DNA can profoundly impact how we experience pain.
Through the lens of genetics, we can unravel the complexities of pain perception, shedding light on why some individuals are more resilient to pain while others are more vulnerable. Let's look at the role of genetics in pain sensitivity and management.
Genetic Variations Influencing Pain Perception
Research has identified numerous genes associated with pain sensitivity, shedding light on the intricate molecular mechanisms underlying this complex phenomenon. These genes encode proteins involved in neurotransmission, nociception, and inflammatory pathways, all of which play pivotal roles in modulating our perception of pain.

1. Genes Associated with Pain Sensitivity
A complicated attribute impacted by numerous genetic and environmental factors, pain sensitivity is controlled by a network of genes engaged in multiple biological processes.
- One such gene is SCN9A, which encodes the Nav1.7 sodium channel—a critical player in pain signaling. Mutations in SCN9A have been implicated in inherited pain disorders, such as congenital insensitivity to pain (CIP) or erythromelalgia, demonstrating the pivotal role of this gene in modulating pain perception.
- Another gene of interest is COMT (Catechol-O-methyltransferase), which regulates the breakdown of neurotransmitters like dopamine and norepinephrine. Variations in the COMT gene have been associated with differences in pain sensitivity, with certain alleles linked to increased pain perception or reduced pain tolerance.
- The OPRM1 gene, encoding the mu-opioid receptor, influences individual responses to opioid analgesics and plays a crucial role in pain modulation. Polymorphisms in OPRM1 have been linked to variations in opioid efficacy and side effects, highlighting the genetic basis of opioid sensitivity.
- Genes involved in inflammatory pathways, such as TNF-alpha and IL-6, contribute to pain sensitivity by regulating the immune response and neuroinflammation. Variations in these genes have been implicated in chronic pain conditions, such as rheumatoid arthritis and fibromyalgia, underscoring the intricate interplay between genetic factors and inflammatory processes in pain perception.
2. Impact of Genetic Mutations on Pain Threshold
Genetic mutations can exert profound effects on an individual's pain threshold, altering the perception and tolerance of painful stimuli. These mutations may disrupt the function of genes involved in nociception, pain modulation, or inflammatory pathways, leading to either heightened or diminished pain sensitivity.
- The SCN9A gene
One illustrative example is the Nav1.7 sodium channel, encoded by the SCN9A gene, mutations that can result in inherited pain disorders characterized by aberrant pain perception. Mutations in SCN9A can lead to either hypoalgesia, characterized by reduced sensitivity to pain, or hyperalgesia, characterized by increased sensitivity to pain.
For instance, loss-of-function mutations in SCN9A have been associated with congenital insensitivity to pain (CIP), a rare condition where individuals are unable to perceive pain, while gain-of-function mutations can cause erythromelalgia, a condition characterized by severe burning pain in the extremities.
- Genes encoding opioid receptors
Mutations in genes encoding opioid receptors, such as OPRM1, can impact pain threshold by altering the efficacy of opioid analgesics. Variations in OPRM1 have been linked to differences in opioid responsiveness, with certain alleles associated with increased analgesic effects or heightened susceptibility to opioid-related side effects.
These genetic differences in opioid receptor function contribute to inter-individual variability in the pain management outcomes, emphasizing the importance of personalized approaches to opioid therapy based on genetic profiling.
- Genes involved in inflammatory pathways
Mutations in genes involved in inflammatory pathways, such as TNF-alpha and IL-6, can modulate pain threshold by influencing neuroinflammation and sensitization of nociceptive pathways. Variations in these genes have been implicated in chronic pain conditions, where dysregulated immune responses contribute to persistent pain states.
Hereditary Factors in Pain Sensitivity
Observations of pain-related traits within families have long suggested a genetic component to pain sensitivity, sparking interest in elucidating the hereditary factors underlying these observations. Adoption studies have further supported these findings by showing that adopted individuals exhibit pain sensitivity traits more closely aligned with their biological relatives than with their adoptive families.
1. Familial Patterns of Pain Sensitivity
Family studies offer valuable insights into the hereditary transmission of pain sensitivity traits across generations and provide evidence for the interplay between genetic and environmental factors in shaping individual pain experiences.
In familial patterns of pain sensitivity, there is often a notable clustering of pain-related conditions or pain perception traits within families. For example, individuals with a family history of chronic pain conditions, such as migraines, fibromyalgia, or temporomandibular joint disorders, are more likely to experience similar pain conditions themselves.
This familial aggregation of pain disorders suggests a shared genetic predisposition that contributes to the transmission of pain sensitivity traits within families.
2 . Twin Studies and Heritability of Pain Perception
Twin studies have been instrumental in unraveling the genetic and environmental contributions to pain perception by comparing the similarity of pain-related traits between monozygotic (identical) and dizygotic (fraternal) twins.
Monozygotic twins share virtually identical genetic makeup, while dizygotic twins share, on average, 50% of their segregating genes, making them genetically comparable but not as genetically similar as monozygotic twins. Twin studies consistently demonstrate higher concordance rates for pain-related traits among monozygotic twins compared to dizygotic twins.
For instance, studies examining pain threshold, pain tolerance, and pain intensity ratings have shown greater similarity in these traits among monozygotic twins, suggesting that genetic factors play a substantial role in shaping individual differences in pain sensitivity.
Genetic Markers and Pain Management
Advancements in genomic research have paved the way for identifying genetic markers associated with pain sensitivity and response to pain management interventions. Genetic markers are specific DNA sequences or variations that are associated with particular traits or conditions, including pain perception and treatment outcomes.
- Identifying Genetic Markers for Pain Sensitivity
Genome-wide association studies (GWAS) analyze genetic variations across the entire genome to identify associations between specific genetic markers and pain-related phenotypes. These studies have identified numerous genetic loci implicated in pain sensitivity, providing valuable insights into the molecular mechanisms underlying individual differences in pain perception.
Candidate gene approaches focus on specific genes or pathways known to be involved in pain processing and modulation. Researchers select candidate genes based on prior biological knowledge and investigate their association with pain-related phenotypes.
This targeted approach allows for a more in-depth exploration of the role of specific genes in pain sensitivity and can provide mechanistic insights into genetic contributions to pain perception.
- Personalized Medicine and Pain Treatment
Genetic markers offer the potential to tailor pain management strategies to individual patients based on their genetic predispositions. For example, genetic markers associated with opioid metabolism and response can inform opioid prescribing practices, allowing for personalized dosing regimens that account for differences in drug metabolism and sensitivity.
Precision medicine aims to deliver the right treatment to the right patient at the right time by taking into account individual genetic variability, environmental factors, and lifestyle considerations. Genetic markers can also help predict treatment response and identify individuals at risk of adverse drug reactions, enabling proactive interventions to mitigate potential risks.
Challenges and Considerations
Despite the potential benefits of integrating genetic information into pain management, several challenges and considerations must be addressed to realize the full potential of personalized approaches to pain care.
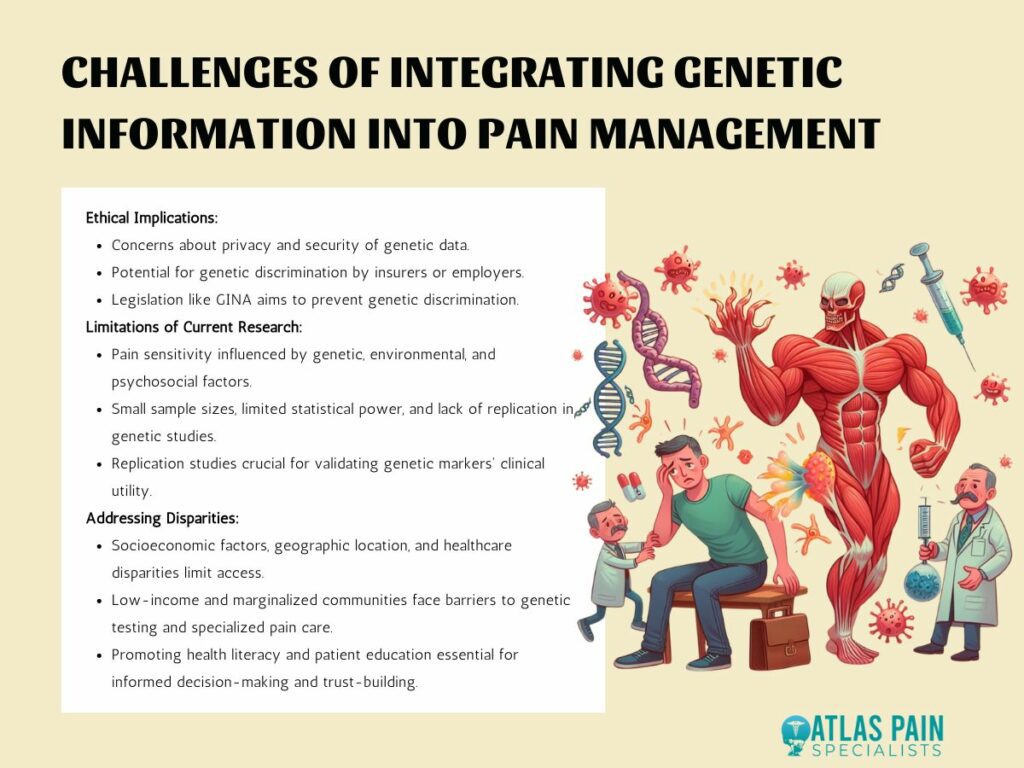
1. Ethical Implications of Genetic Testing for Pain Sensitivity
Genetic testing for pain sensitivity raises concerns about privacy and the security of genetic data. Patients may be hesitant to undergo genetic testing if they fear that their genetic information could be misused or accessed without their consent.
Genetic information revealing predispositions to pain sensitivity or susceptibility to certain pain conditions may potentially be used by insurers or employers to discriminate against individuals. Legislation such as the Genetic Information Nondiscrimination Act (GINA) in the United States aims to prevent genetic discrimination in health insurance and employment.
2. Limitations of Current Research in Genetics and Pain Management
Pain sensitivity is influenced by a complex interplay of genetic, environmental, and psychosocial factors. While genetic markers provide valuable insights into the genetic basis of pain perception, they represent only one piece of the puzzle.
Many genetic studies investigating pain sensitivity and treatment response suffer from small sample sizes, limited statistical power, and insufficient replication of findings. Replication studies in independent cohorts are crucial for validating the association between genetic markers and pain-related phenotypes and establishing their clinical utility.
3. Addressing Disparities in Access to Genetic Testing and Personalized Pain Care
Access to genetic testing and personalized pain care may be limited by socioeconomic factors, geographic location, and healthcare disparities. Low-income individuals and marginalized communities may face barriers to accessing genetic services and specialized pain management interventions.
Promoting health literacy and patient education is essential for empowering individuals to make informed decisions about genetic testing and personalized pain management. Educating patients about the benefits, limitations, and implications of genetic information can help facilitate shared decision-making and foster trust between patients and healthcare providers.
Debunking Pain Management Myths
Genetic markers offer insights into individual differences in pain perception, guiding personalized approaches to pain management that prioritize efficacy and minimize adverse effects. However, as we navigate the integration of genetic information into clinical practice, it is imperative to address ethical considerations, overcome research limitations, and ensure equitable access to genetic testing and personalized care.
By debunking misconceptions surrounding pain and its treatment, we pave the way for a more informed understanding of pain management, including the role of genetics. Through education and awareness, individuals can make informed decisions about genetic testing and personalized pain care, empowering them to actively participate in their treatment journey.
Debunking myths fosters trust between patients and healthcare providers, facilitating open communication and collaborative decision-making in pain management. Ultimately, by combining scientific advancements in genetics with accurate information and patient-centered care, we can strive towards more effective and personalized approaches to pain management, enhancing the quality of life for individuals living with pain.
About Dr. Sean Ormond